Drug Factory Error Triggers Werewolf Syndrome in 17 Infants
A pharmaceutical mishap by Málaga-based company Farma-Química Sur resulted in the accidental distribution of a vasodilator drug intended for treating alopecia as omeprazole in bulk quantities.
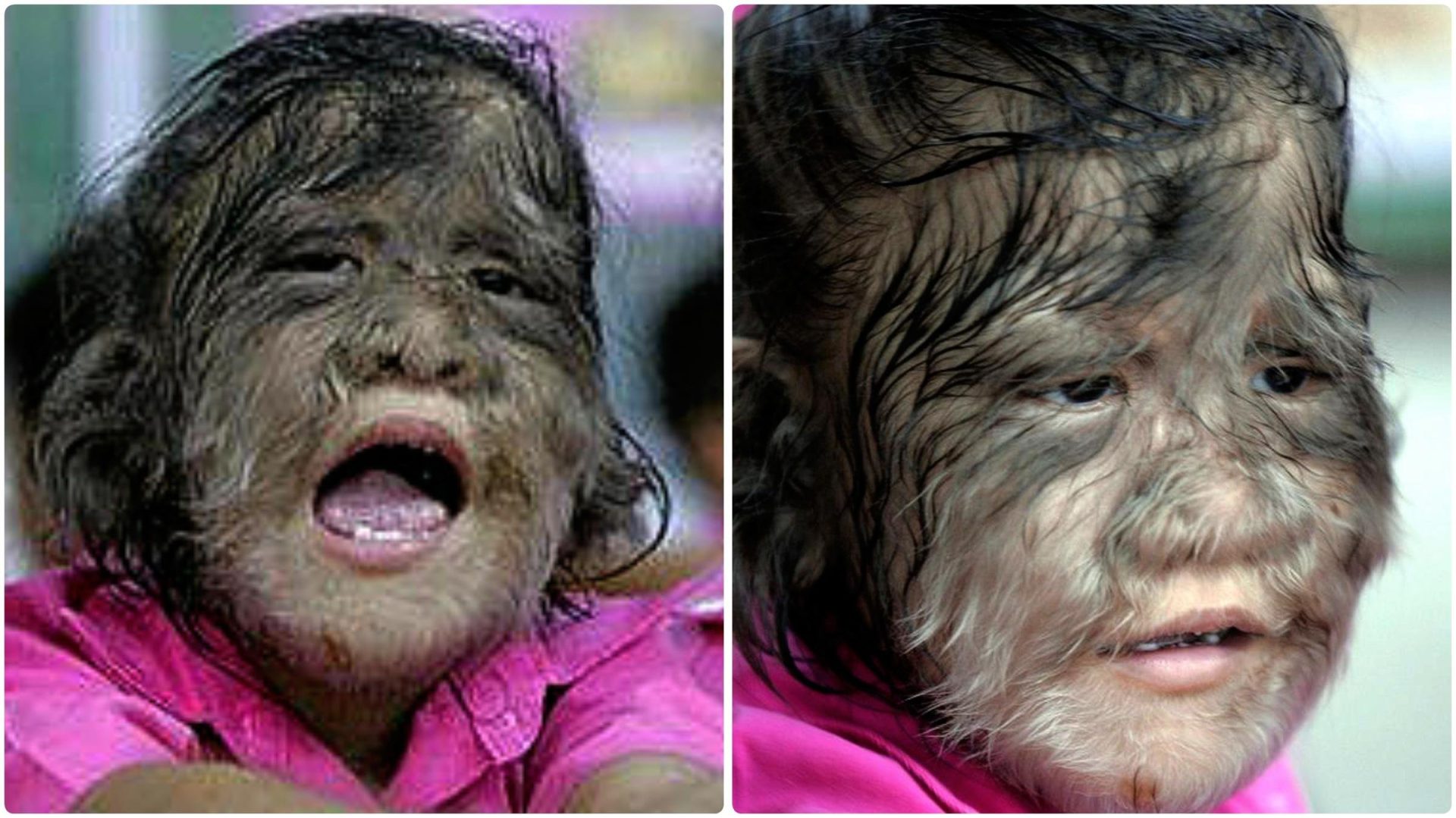
This internal mistake led to the inadvertent marketing of minoxidil, a potent vasodilator used to treat alopecia, under the guise of omeprazole. As a consequence, individuals who consumed this wrongly labeled medication developed hypertrichosis, commonly known as the werewolf syndrome.
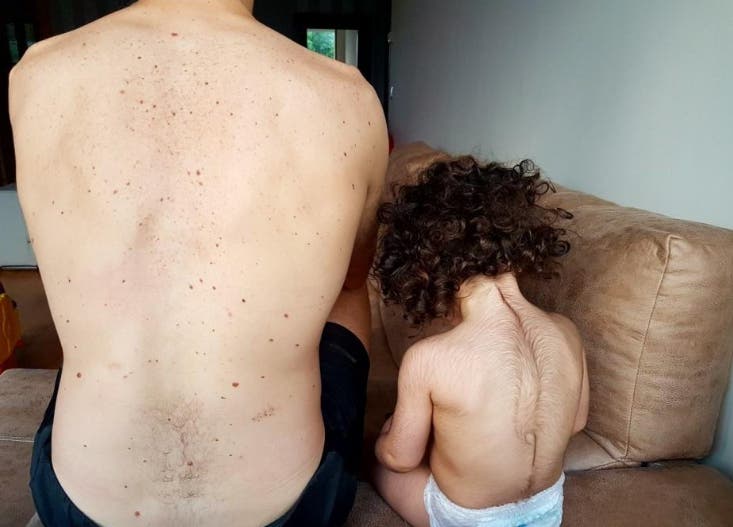
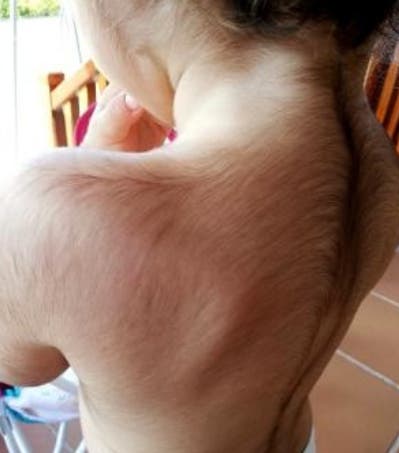
Ángela Selles, the mother of Uriel, one of the affected infants, shared her distress, recounting how her son developed excessive hair on his forehead, cheeks, arms, legs, and even hands. A total of 17 affected babies have been identified in Spain, according to the Spanish Agency for Medicines and Health Products (AeMPS).
Initially, doctors reporting cases to the Spanish System of Pharmacovigilance of Medicines for Human Use (SEFV-H) brought this alarming issue to the attention of AeMPS. The investigation led inspectors to Farma-Química Sur's factory in Malaga.
Upon examining the bulk omeprazole from India, they found it to be unaffected, but smaller batches sold in bulk exhibited the error. This confusion stemmed from the packaging indicating omeprazole when it was actually minoxidil.

Werewolf syndrome results in excessive hair growth across the body. The AeMPS, an entity under the Ministry of Health, discovered another affected child in Granada. Ten cases were reported in Cantabria, four in Andalusia, and three in the Valencian Community.
The parent of a child who developed the syndrome, choosing to remain anonymous, expressed their distress upon witnessing the changes in their three-month-old baby. Specialist consultations became necessary to eliminate the possibility of various syndromes or rare conditions.
On July 11, AeMPS issued an alert, which was expanded on August 6 due to the identification of new cases of children diagnosed with hypertrichosis. The factory has been closed due to severe breaches in drug manufacturing controls. The company has six months to address the reported errors and implement corrective measures for potential factory reopening.

Doctors noted that the excess hair growth in affected children may persist for months. The impact of minoxidil's active ingredient on patients, particularly young ones, is being investigated. The most severe potential side effect is related to cardiac disorders.
The Cantabria Prosecutor's Office launched an investigation in early August following criminal complaints from four families.

It's important not to leave without sharing the details of this incident that has impacted many families. We will continue to provide updates on the progress of this case.



